Professor Chris Butler is the lead investigator on the much delayed and recently published study on Ivermectin. We have waited years for this study to complete, by which time, its relevance has dissolved away to near zero.
I don’t want to spend time going through the detailed analysis that’s already been done on this study - for a really detailed look at this, check out
and his article about it. Needless to say, it was perfectly designed to fail. Have a look at the way the odds were stacked against Ivermectin in this trial in this great comparison done by the people at c19ivm.org.On the left we see molnupiravir, a darling drug of the pharmaceutical industry that costs £513 for a course of treatment. On the right is the ‘open source’ drug Ivermectin, which would cost perhaps £25. The investigator is looking for evidence of the drugs usefulness for treating Covid-19. The table is comparing the methods used in a study that intended to investigate each drugs effectiveness. Do you notice anything about the table? The first thing to draw your attention to is that both studies were run by Chris Butler.
The ‘delay’ is the period of time waited before the treatment was started on the patients. Treatment for the ivermectin study might have started up to fourteen days after symptom onset. Now I don’t know about you, but there’s a very good chance you’re already well on the mend by then, so any effect you might observe in the treated patients will have now very likely have been missed. It’s like turning up to a restaurant with a stomach full of food and then blaming the restaurant for your lack of an appetite.
See the population? The molnupirair study focused on either older people (more likely to get sick), or people with comorbidities (more likely to get sick), whilst the Ivermectin population were just healthy people from 18 up. Why? Well, sick people benefit more from interventions than healthy people, so if you stack your population with sick people you can amplify the measured effect compared with a population that a) are already healthy, b) may have already recovered by the time you administer treatment.
Are you getting it yet?
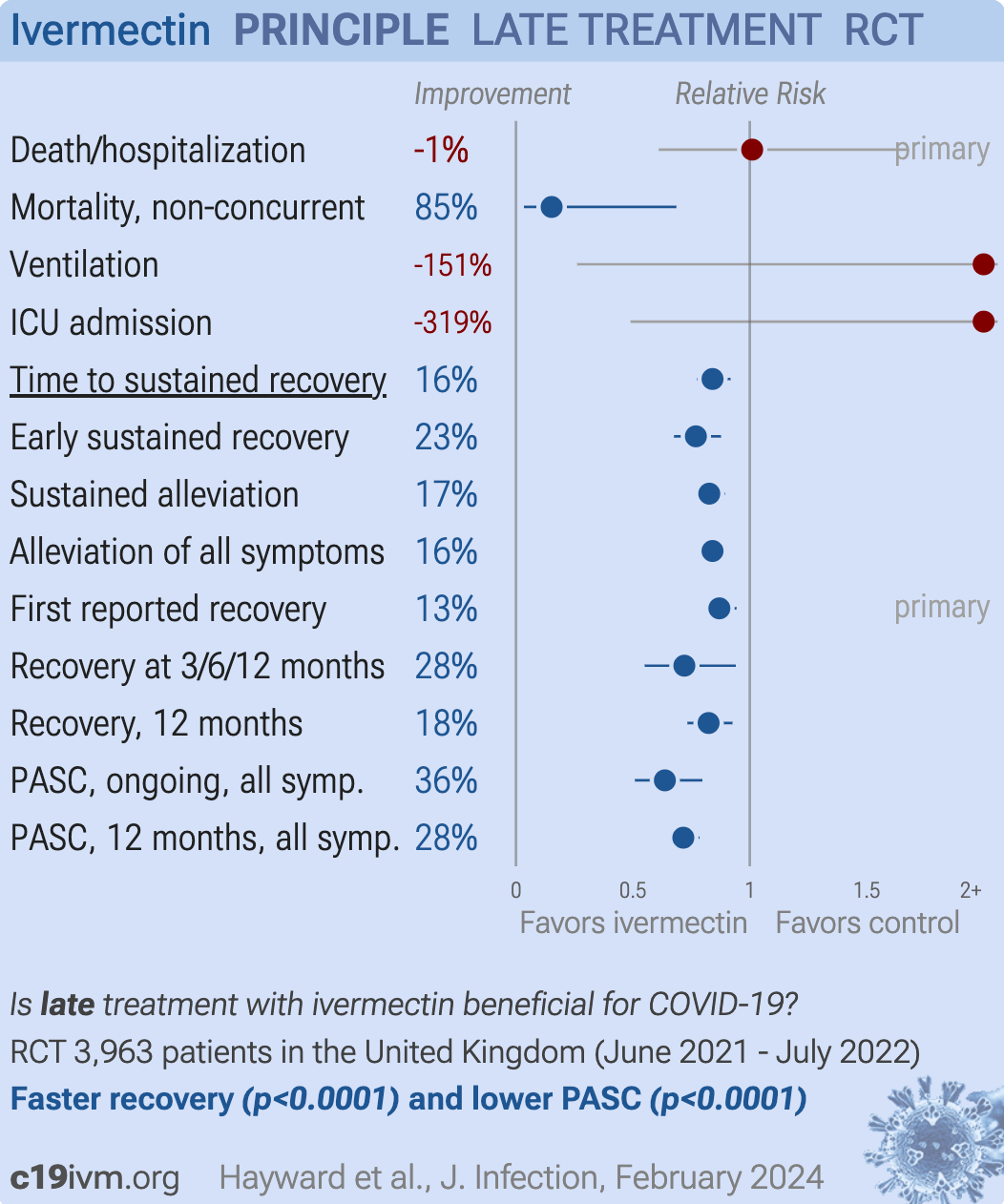
They cooked the books, so I won’t labor the point. Even then after all these enraging tricks, the investigators still couldn’t bury the positive effect of Ivermectin. Time to recovery was significantly improved, alleviation of symptoms significantly improved, first reported recovery significantly improved. I won’t go on, you can look at the graph yourself; anything where the line and dot do not touch the central vertical line is a good result.
So with all this data, despite the deliberate roadblocks the investigators had set, what was the conclusion in this Ivermectin study? “Ivermectin for COVID-19 is unlikely to provide clinically meaningful improvement in recovery”.
What?
This trickery is something that actually happens regularly, where the written conclusions do not match the data in the actual study. Meanwhile, what do you think the conclusion of the study into molnupiravir was? Despite the data being…bad, the authors bend over backwards to speak politely about the drug, with this sentence being indicative of the mental gymnastics taking place, “The primary analysis estimated a 33% probability of superiority—that is, there is a 33% chance that the addition of molnupiravir to usual care reduces hospitalisation or death by any non-zero amount.” This absurd line of reasoning was even parodied way back in 2004 by Paul Rudd in Anchorman.
Only the absurdities here are even worse: 33% of the time, it works a ‘non-zero’ amount. Later in the same study, the authors write “plausible effects for molnupiravir could range from a 19% reduction to a 41% increase in the risk of hospitalisation or death.”
I’ll leave you to mull over the absurdities of that, but please, really do mull over just how utterly absurd that is.
So how do we end up here? Where absolutely everything is done to save the face of a useless darling drug whilst absolutely everything is done to tarnish the reputation of a useful and cheap one? Well, the conflicts of interest section at the end of these papers can help paint that picture. On the molnupiravir study, if we run the declaration of interests section through an AI we can match up the frustrating name abbreviations with the study authors, and this is what we get.
Prof Jonathan S Nguyen-Van-Tam (JSN-V-T): Seconded to the Department of Health and Social Care, England from October 2017 to March 2022. Received lecture fees from Gilead and fees for participation on an advisory board for F Hoffmann-La Roche.
Prof Kerenza Hood (KH): Member of the Health Technology Assessment General Committee and Funding Strategy Group, and Research Professors Funding Committee at the UK National Institute for Health and Care Research (NIHR). Received a grant from AstraZeneca (paid to their institution) for a trial of Evusheld for the prevention of COVID-19 in high-risk individuals. Independent member of the data monitoring committee for the OCTAVE-DUO trial of vaccines in high-risk individuals.
David M Lowe (DML): Received grants or contracts from LifeArc, the UK Medical Research Council, Bristol Myers Squibb, GlaxoSmithKline, the British Society for Antimicrobial Chemotherapy, and Blood Cancer UK. Personal fees or honoraria from Biotest UK, Gilead, and Merck. Consulting fees from GlaxoSmithKline (paid to their institution). Conference support from Octapharma.
Prof Duncan B Richards (DBR): Received consulting fees from OMASS Therapeutics. Leadership and fiduciary role in the Heal-COVID trial TMG.
Benjamin R Saville (BRS), Joe Marion (JM), Melissa A Detry (MAD), Christina T Saunders (CTS), Nicholas S Berry (NSB), Mark Fitzgerald (MF): Report grant money paid to their employer (from who?) from the University of Oxford for the statistical design and analyses of the PANORAMIC trial. JM also participated on data and safety monitoring boards as part of his employment with Berry Consultants.
Mark Lown (ML): Member of the data monitoring and ethics committee of RAPIS-TEST (NIHR efficacy and mechanism evaluation).
Prof Saye Khoo (SK): Reports grants from GlaxoSmithKline, ViiV, Ridgeback Biotherapeutics, Vir, Merck, the UK Medical Research Council, and the Wellcome Trust (all paid to his institution). Speaker's honoraria from ViiV. Donations of drugs for clinical studies from ViiV Healthcare, Toyama, and GlaxoSmithKline.
Prof Joseph F Standing (JFS): Participated on a data safety monitoring board for GlaxoSmithKline.
Prof Mahendra G Patel (MA): Received grants from various organizations (paid to their institution) and consultancy fees from Prenetics and OxDx. Planned patent for Ramanomics. Participation on data safety monitoring boards or advisory boards for Prenetics. Unpaid leadership or fiduciary role in the E3 Initiative.
Nicholas P B Thomas (NPBT): Payment for participation on an advisory board from MSD.
Oliver van Hecke (OvH): Received consulting fees from MindGap (fees paid to Oxford University Innovation). Participation on data safety monitoring boards or advisory boards for the CHICO trial. Unpaid leadership or fiduciary role in the British Society of Antimicrobial Chemotherapy.
Prof Andrew Ustianowski (AU): Received consulting fees and payment or honoraria from MSD, GlaxoSmithKline, and Gilead.
Nicholas Francis (NF): Received consulting fees from Abbott Diagnostics and GlaxoSmithKline. Member of the PRINCIPLE trial data safety monitoring board and the NIHR Health Technology Assessment General Funding Committee. Holds stocks in Synairgen.
Prof Judith Breuer (JB): Received consulting fees from GlaxoSmithKline (paid to her institution).
Does that look like the fluffy realm of benevolent ‘science’ you might have imagined it to be? Knowing the authors of these papers have this many conflicts of interest should brings into focus the enormity the problem we have in medical science. Our doctors, knowingly or unknowingly, are dependent on a system of intelligence gathering which is bought and paid for by a pharma cartel.
Shall we get into it? Because even here, on this one study, there’s so much to unpack, and all of it will pour light onto the absurdity that is modern pharmaceutical ‘science’. So please, get a paid subscription to The Digger and please share the article where you can. For long time readers of The Digger, you’ll know that (thanks to my paid subscribers) there’s now an incredible AI tool behind us at case.science. It’s a tool that’s truly needed if we’re ever going to overturn this absurd publishing system. I’ll be getting right back into the good stuff of who and what is really driving our medical system. Subscribe, there’s more to come.







Bravo. Have noted Steve Kirsch is suing the journal Springer Nature for the unethical retraction of their paper, “COVID-19 mRNA Vaccines: Lessons Learned from the Registrational Trials and Global Vaccination Campaign.” Now for pushback against the war on repurposed drugs supported by these nonsensical ‘studies’.
I do wonder how people like Chris butler sleep at night. I emailed him and told him this as well as asking a few obvious questions. It sickens me that rogue “professors” get away with shoddy science. Sadly, we have seen it all before. Get your ivermectin, keep it in your medicine cabinet and by all means use it when needed. Thanks Chris you reinforced my trust in ivermectin and in all the brave scientists, researchers, data analysts, legal experts who have but their reputations on the line to find humanity an affordable and safe treatment for covid. You sir did the opposite, shame on you!