The 'very unusual paper.' Part 1
The incentives of Ivermectin's 'ghostwriters'. Was an effective medicine moved out the way?
For the incredibly popular ‘summary’ on Ivermectin, click here. For a deeper dive into Ivermectin, read on.
***
Whenever I create anything, the first thing I obsess over is ‘who is this for?’ This is a series of articles that detail the Ivermectin scandal, how and why it appeared as a potential treatment, why it disappeared and who played a role in that.
This part is for those who are fresh to the controversy over Ivermectin. If ‘the Ivermectin chat’ has popped up in your silo of socials, this article is written to serve as an effective jump-off point - so please do share it. There’s an audience I hope it can reach, so if you’re aware of friends or family in that audience, it would be great if you’d help the article find that audience.
Ivermectin is controversial, but I hope to illuminate what the controversy is. You may be about to read something you disagree with - perhaps you’re hostile to it - but please remember I aim to inform that disagreement. If you’ve not looked closely at Ivermectin, or the mention of it gives you the heeby-jeebies, I hope the article can at least inform those heeby jeebies. Data is not political, please, do not get drawn into politicising drugs and the data that support them.
Why did I write this? Because what happened exactly 12 months ago was not properly documented at the time. Things were pretty crazy back then and we are still dealing with the fallout from that moment. Regardless of what you think about Ivermectin, what I’m about to detail is worthy of attention. For those who’ve followed the Ivermectin story carefully, there’s something very new for you here in parts two and three, where I get into the who, when, and why…
Ivermectin Part 3: The People Behind the Curtain
Before we go any further into a discussion on Ivermectin, I need to dislodge a nugget of disinformation that may have lodged into your psyche.
Because of a now infamous tweet by the FDA, when people hear the word ‘Ivermectin’, they think ‘horse dewormer’. Characterizing Ivermectin as a horse dewormer is unfair, but it’s better described as a lie.
Ivermectin is considered by the World Health Organisation as a vital global medicine. It was first brought to market in 1987 as an anti-parasitic and has since been administered - in humans - more than 3 billion times. Over 30 years of use, Ivermectin has built a reputation as a remarkably safe drug. Up there with asprin or paracetamol. Its miraculous successes in ridding the world of river blindness and elephantiasis - in humans - led its discoverer, Satoshi Ōmura, to jointly win the Nobel Prize in 2015. The first research suggesting Ivermectin may also have anti-viral properties turned up in 2012. It’s therefore quite reasonable to see Ivermectin as a candidate in the fight against COVID. In humans.
I’m sorry I had to write that - but here we are. If you’d never really paid much attention to Ivermectin but you still thought ‘horse paste’ when you heard the word, it’s worth considering how such a thing might have happened. Why would a regulator try to make the public associate a Nobel Prize-winning drug with horse worms?
Now that’s out of the way, we can get started.
An exciting discovery
In April 2020, Kylie Wagstaff and her team at Monash University made an amazing discovery: a drug called Ivermectin killed the Sars-COV-2 virus in vitro. That means the effect was demonstrated in a lab, not a human or an animal. An academic debate followed; some said the high dose required meant it would never work in humans, others argued high doses wouldn’t be needed. To settle that debate, we needed good observational and randomized control trials that looked for an effect in humans.
Could it be? That a cheap, safe, highly available drug could work to mitigate the worst effects of coronavirus? Very quickly, the research world stepped up to the plate.
Studies cropped up all over the world: Italy, Egypt, Iraq, Argentina, Columbia, Mexico and Spain. Nowhere was safe from the virus of knowledge expansion! Researchers begged for small grants or sometimes got nothing at all as they worked tirelessly to build our knowledge on this issue. Running parallel to the infamous research into vaccines, this was the other incredible COVID research effort that most people never heard of. By the end of July 2020, there were more than 32 studies registered investigating Ivermectin’s effectiveness against Sars-COV-2.
Incredibly, the first randomized control trial published its findings in July 2020. It can be credited to Dr Mohiuddin and his team in Bangladesh - a randomized study of 116 patients. The result? Ivermectin lowered the risk of being hospitalized with COVID. It was the first of many studies to start producing results.
At that exact moment, Dr Andrew Hill of the University of Liverpool had been investigating repurposed drugs in the fight against COVID-19. With such good data arriving on Ivermectin, Hill and his team dropped their investigations into other drugs. Guided by World Health Organisation criteria, they put together a ‘systematic review’ of the growing body of randomized control trials looking into Ivermectin. A systematic review is amongst the highest level of evidence to support a drug’s effectiveness. With that in mind, take two minutes to see where the team were as early as December 2020.
https://rumble.com/vu17ze-andrew-hill-presents-to-collaborative-covid-workshop-december-2020.html
Hill’s team had already found a strong signal showing Ivermectin was effective, and more data was coming in over the next few months. He wasn’t alone - a few weeks earlier Dr Piere Kory testified before the US Senate saying the same thing. Some weeks later, Dr Andrew Hill and Dr Pierre Kory together would share their evidence with the National Institute of Health in the USA - the main agency responsible for biomedical and public health research.
https://rumble.com/vtw9ip-pierre-kory-testifies-to-the-us-senate-december-8th-2020.html
By now, there was another top researcher also reviewing the evidence for Ivermectin: Dr Tess Lawrie. Given that thousands of people were dying, Lawrie rightly considered it an emergency to get a systematic review completed. By 6th January 2021, her rapid review of the evidence also found a strong signal of benefit, “Ivermectin substantially reduces the risk of a person dying from COVID-19 by probably somewhere in the region of 65% to 92%."
Three researchers. All independent. All found a signal.
When I say they ‘found a signal’ - what do I mean? Let’s say researchers are looking at studies where doctors tried using Ivermectin against COVID. Generally, the study is comparing the results of two groups: those that received Ivermectin and those that didn’t. They measure what happened in those two groups. How fast did they get better? How quickly did they clear the virus? How many people died? And so on.
The researchers call those outcomes ‘endpoints’, and they’re often identical across multiple independent studies looking into the same thing. If you were studying ‘dog cuteness’, you’d likely measure ‘eye size’ and ‘coat fluffiness’ and you’d be very likely to find these identical endpoints across many studies into dog cuteness. Now you know what endpoints are.
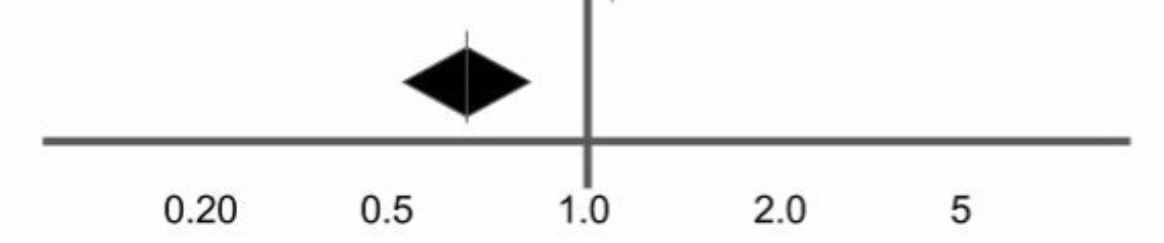
In a systematic review, the most important ingredient is the studies themselves - trials run by independent researchers where one group of patients are given Ivermectin and the other group are given a placebo. Grassroots research teams from all over the world really delivered here. From that pool of research, you select the best studies available, add together all the endpoints and then weight them, usually based on the study size (more patients in the study gives more weight). When you’re done you get a forest plot, an interesting diamond shape that gives you your answer. A forest plot is like looking at your signal. A diamond on the left means drug works, diamond on the right means the opposite. The smaller the diamond, the more confident the finding. Crucially, when the diamond does not touch the line in the middle - that’s ‘a signal’. It’s a way to ‘tune in’ noisy data and hear what it says. So this is what looking at a signal looks like!
Below are the results of the three independent systematic reviews into Ivermectin and its effect on mortality. Essentially answering the question: does it reduce your risk of dying from COVID? Now you know how to read a forest plot, so go ahead and see if you can see a signal. Each time, pay attention to the ‘total’ or ‘overall’ line with the black diamond at the bottom - that’s all the results added together. If it doesn’t touch the middle line, it’s a signal. You can also see a summary of the studies they included in their review.
Dr Piere Kory.
Pre-print, later peer-reviewed and published, December 2020:
Dr Andrew Hill.
Lecture on preliminary findings in December 2020:
Dr Tess Lawrie.
Rapid review in January 2021
By January 2021, three independent researchers, each looking at the available data on Ivermectin, all found the same signal: Ivermectin works against Sars-COV-2. All three researchers were confident the data was good, they knew the drug was safe, and they knew the drug was cheap and readily available. In his December 2020 lecture, Hill said: “I've been working on repurposed drugs right since the beginning. I haven't seen any data like this, data so consistent between such a wide range of countries, all looking at the data in slightly different ways but getting quite consistent conclusions…Even if we hit 48% improvement, which looks like the worst case, that would still be a major advancement in treatment.”
By now, the ‘sample size’ supporting Ivermectin, which you get by adding together all the patients in all the studies, was 2,294. The approval of Remdesivir in the USA, a drug that costs $2,200 a dose, has a terrible safety profile and arguably zero clinical benefits, was approved on a single industry-sponsored trial involving 1,062 people. With double the sample size, a proven safety record, and high availability at $3 a treatment, wasn’t it time for Ivermectin to get approved?
So what happened? Press on, dear reader…
Dr Tess Lawrie added to the momentum by sharing her findings with the UK authorities and other agencies around the world, but it was Hill’s study that looked most likely to create policy change. In the clip above, Hill said his work was sponsored by Unitaid. They’re an organisation ‘hosted’ by the World Health Organisation. We’ll return to Unitaid in part two. He said the work ‘could get to a WHO recommendation for the treatment being used worldwide’. The paper itself had not yet been published, but Hill had been sharing the preliminary findings at the NIH and various panels.
Getting the World Health Organisation to pay attention to systematic reviews is an area of expertise for Dr Tess Lawrie. Given this expertise, and the obvious crossover in their work, Hill and Lawrie started to collaborate. They decided how they would structure the paper, which studies would be included, and how they would grade the evidence using the ‘Cochrane’ method - a highly respected method of systematically reviewing scientific data. By 15 January 2021, their collaboration was looking very productive and they were both keen to present the data in the best possible manner to get the WHO recommendation. Privately, both knew it was just a matter of time. With the data they had, Ivermectin would be approved.
What had been a fruitful professional relationship suddenly fell apart. Hill jumped the gun. Totally out of the blue, he published his research paper without Lawrie.
“It was very unusual,” Lawrie later said.
The paper defied many norms in medical publishing. When you conduct a systematic review, you’re assessing how biased and bad the studies are, but for reasons unknown, Hill had included all of the studies’ authors as co-authors of his paper. To those in the know, this is like turning up to a cricket match with a baseball bat. The strange grammar of the paper was just the start of the oddities; the main confusion was over the conflicting statements. First, the good news: when using Ivermectin in moderate or severe COVID cases ‘…there was a 75% reduction in mortality..with favourable clinical recovery and reduced hospitalisation’.
Great! At that moment, 15,000 people were dying each day, so the data suggested Ivermectin might save 11,250 people per day if used early. But, as you’re probably aware, this isn’t how the story ends…
In the discussion section, Hill performed a puzzling about-turn: ‘Ivermectin should be validated in larger appropriately controlled randomized trials before the results are sufficient for review by regulatory authorities’ [emphasis added]. That’s right. The researcher working tirelessly to get the World Health Organization to recommend Ivermectin had instead - for reasons unknown - argued to not use Ivermectin. The paper was ringing alarm bells.
“The long author list… [the fact that] he didn’t grade the evidence… As scientists, we don’t usually make policy recommendations, we just present our data. It was all very unusual, '' Lawrie said. The policy switcheroo was just one of her concerns. Hill also hadn’t graded his evidence to demonstrate the certainty of the effect. This seriously downplayed the strength of the findings. Remember those signals we could see in the forest plots earlier? That certainty should have been communicated in the conclusion, and it wasn’t. Instead, there was a policy recommendation not to use Ivermectin.
Dr Piere Kory and his colleague Dr Paul Marik could also see red flags. To those familiar with scientific papers, they stood out a mile. “The limitations section is at the end of the introduction and the end of the paper… The mortality benefit was mentioned last, not first… Repeated mentions of Ivermectin having no antiviral activity, which goes against claims he already made… I have never heard a doctor, or a researcher, talk about ‘what is sufficient for review by regulatory authorities. No doctor writes that.”
To add to the confusion, the Financial Times ran a story on the paper the day it was published. They too quoted the finding that it reduced mortality by 75%, and even quoted Hill himself. “The purpose of this report is to forewarn people that this is coming: get prepared, get supplies, get ready to approve it… we need to be ready.” In the days that followed, Andrew remained as bullish about Ivermectin as ever. In a discussion with a French publication called Bons Sens, Hill excitedly described the dose-dependent effect: “We are seeing very clear anti-viral effects. We see smaller effects when the drug is given for one day, then in dose-ranging studies, we see more and more of an effect. And then if the drug is given at a high dose, for five days, we see the strongest effect. So, how could that be happening if the drug does not stop the virus from replicating? It simply does. It does. And we’ve got the evidence to prove it…. It’s just a matter of time before it gets approved.”
Hill had the evidence to approve it, and it was just a matter of days. So what was going on? It was as though there were two Dr Andrew Hills: one a public advocate for Ivermectin seeking a WHO recommendation, and another who argued against using Ivermectin and against the WHO recommending it. How could this be reconciled? Was someone influencing the research paper? Was such a thing possible?
Dr Kory certainly thought so. “From his previous presentations and private conversations, we knew that someone else had influenced his paper… something happened to that paper.”
Dr Lawrie was concerned, too. She knew how the paper would be received, and that its bizarre policy recommendation and the lack of graded evidence was going to leave them dead in the water. She emailed him right away. “This review is going to cause immeasurable harm.” she wrote. “Can you please retract it and we can do it properly?” To her surprise, Hill answered. He wanted to talk. On 18 January 2021, they spoke via Zoom.
Below is a transcript of the conversation. It’s worth reading in full. Hill, under intense questioning, admitted that Unitaid - the study’s sponsor - had a say in the conclusions of the paper. He said the paper had been influenced by persons unknown, and the net effect was that Ivermectin was never adopted globally despite the evidence. Sometime after that, you would come to associate the drug with worms riggling around inside the guts of horses.
Lawrie wanted to know: which people at Unitaid influenced the paper? ‘Just the people there, I’m not going to start naming names’ Hill replied. ‘It’s a consensus.’
We know that Hill had been ‘reporting into a team at Unitaid’, as he’d told Bons Sens exactly that. If ‘people’ at Unitaid had helped write the conclusion of the paper, perhaps even all of the paper, those ‘people’ ought to be listed as authors, but they are not listed. We don’t even know their names, nor their motivations. They are ghostwriters, shaping the paper in their own particular way. In 2020, we had a therapy for COVID that looked likely to have saved thousands of lives. Instead, a faceless group weakened the data in an underhand way.
But why? Why would Unitaid - why would anyone, for that matter - want to put the brakes on the ‘proof’ that Ivermectin was an effective treatment for COVID during a pandemic? Such a thing would be unthinkable, wouldn’t it?
There is an answer to that question. The why is where things get really interesting, and I’ll detail it all in part two. If you’re tempted to write ‘but research fraud!’ in response to an article showing ghostwriters on critical COVID science, at least wait until we get to part two.
Ivermectin Part 3: The People Behind the Curtain
NOTE
I do not yet have the full video from where this transcript was taken, so this is a transcript I am relying on. I can confirm the recording is real - I’ve seen parts of it - and that the transcript is accurate according to what I have seen. I’ve spoken with Dr Tess Lawrie and the custodians of the video and they confirm it is real and that the following words were really spoken.
Before you read it, I want to warn you that it may make you quite angry. Do not, under any circumstances, let that anger direct your actions. Do not try to contact Dr Andrew Hill. I have met with Andrew, and he is a pleasant man. I believe he is perhaps stuck in something beyond his control. For the record, he denies that he was unduly influenced and stands by his research, saying that he’d been warned in December that large-scale scientific fraud on Ivermectin was taking place - a claim I will test in part two of this series. I contacted Unitaid for comment, giving ample time for a response, but they failed to respond.
The conversation was recorded via Zoom, and according to Zoom’s policy, all participants are notified when calls are being recorded and it is not possible to turn this feature off.
Please support me on Substack so I can continue to do this work.
The Transcript
Dr Andrew Hill: "I mean, I, I think I'm in a very sensitive position here...."
Dr Tess Lawrie, MD, PhD: "Lots of people are in sensitive positions: they're in hospital, in ICUs dying, and they need this medicine."
Dr Andrew Hill: "Well...."
Dr Tess Lawrie: "This is what I don't get, you know, because you're not a clinician. You're not seeing people dying every day. And this medicine prevents deaths by 80 percent. So 80 percent of those people who are dying today don't need to die because there's Ivermectin."
Dr Andrew Hill: "There are a lot, as I said, there are a lot of different opinions about this. As I say, some people simply...."
Dr Tess Lawrie: "We are looking at the data; it doesn't matter what other people say. We are the ones who are tasked with... look[ing] at the data and reassur[ing] everybody that this cheap and effective treatment will save lives. It's clear. You don't have to say, well, so-and-so says this, and so-and-so says that. It's absolutely crystal clear. We can save lives today. If we can get the government to buy Ivermectin."
Dr Andrew Hill: "Well, I don't think it's as simple as that, because you've got trials...."
Dr Tess Lawrie: "It is as simple as that. We don't have to wait for studies... we have enough evidence now that shows that Ivermectin saves lives, it prevents hospitalization. It saves the clinical staff going to work every day, [and] being exposed. And frankly, I'm shocked at how you are not taking responsibility for that decision. And you still haven't told me who is [influencing you]? Who is giving you that opinion? Because you keep saying you're in a sensitive position. I appreciate you are in a sensitive position, if you're being paid for something and you're being told [to support] a certain narrative... that is a sensitive position. So, then you kind of have to decide, well, do I take this payment? Because in actual fact, [you] can see [your false] conclusions... are going to harm people. So maybe you need to say, I'm not going to be paid for this. I can see the evidence, and I will join the Cochrane team as a volunteer, like everybody on the Cochrane team is a volunteer. Nobody's being paid for this work."
Dr Andrew Hill: "I think, fundamentally, we're reaching the [same] conclusion about the survival benefit. We're both finding a significant effect on survival."
Dr Tess Lawrie: "No, I'm grading my evidence. I'm saying I'm sure of this evidence. I'm saying I'm absolutely sure it prevents deaths. There is nothing as effective as this treatment. What is your reluctance? Whose conclusion is that?"
[Hill then complains again that outsiders are influencing him.]
Dr Tess Lawrie: "You keep referring to other people. It's like you don't trust yourself. If you were to trust yourself, you would know that you have made an error and you need to correct it because you know, in your heart, that this treatment prevents death."
Dr Andrew Hill: "Well, I know, I know, for a fact that the data right now is not going to get the drug approved."
Dr Tess Lawrie: "But, Andy - know this will come out... It will come out that there were all these barriers to the truth being told to the public and to the evidence being presented. So please, this is your opportunity just to acknowledge [the truth] in your review, change your conclusions, and come on board with this Cochrane Review, which will be definitive. It will be the review that shows the evidence and gives the proof. This was the consensus on Wednesday night's meeting with 20 experts."
Dr Tess Lawrie: "Yeah, because the NIH is owned by the vaccine lobby."
Dr Andrew Hill: "That's not something I know about."
Dr Tess Lawrie: "Well, all I'm saying is this smacks of corruption and you are being played."
Dr Andrew Hill: "I don't think so."
Dr Tess Lawrie: "Well then, you have no excuse because your work in that review is flawed. It's rushed. It is not properly put together….This is bad research... bad research. So, at this point, I don't know... you seem like a nice guy, but I am really, really worried about you."
Dr Andrew Hill: "Okay. Yeah. I mean, it's, it's a difficult situation."
Dr Tess Lawrie: "No, you might be in a difficult situation. I'm not, because I have no paymaster. I can tell the truth... How can you deliberately try and mess it up... you know?"
Dr Andrew Hill: "It's not messing it up. It's saying that we need, we need a short time to look at some more studies."
Dr Tess Lawrie: "So, how long are you going to let people carry on dying unnecessarily - up to you? What is the timeline that you've allowed for this, then?"
Dr Andrew Hill: "Well, I think… I think that it goes to WHO and the NIH and the FDA and the EMEA. And they've got to decide when they think enough's enough."
Dr Tess Lawrie: "How do they decide? Because there's nobody giving them good evidence synthesis because yours is certainly not good."
Dr Andrew Hill: "Well, when yours comes out, which will be in the very near future… at the same time, there'll be other trials producing results, which will nail it with a bit of luck. And we'll be there."
Dr Tess Lawrie: "It's already nailed."
Dr Andrew Hill: "No, that's, that's not the view of the WHO and the FDA."
Dr Tess Lawrie: "You'd rather... risk loads of people's lives. Do you know if you and I stood together on this, we could present a united front and we could get this thing. We could make it happen. We could save lives; we could prevent NHS doctors and nurses people from getting infected. We could prevent the elderly from dying."
Dr Tess Lawrie: "These are studies conducted around the world in several different countries. And they're all saying the same thing. Plus there's all sorts of other evidence to show that it works. Randomized controlled trials do not need to be the be-all and end-all. But [even] based on the randomized controlled trials, it is clear that Ivermectin works... It prevents deaths and it prevents harm and it improves outcomes for people...
I can see we're getting nowhere because you have an agenda, whether you like it or not, whether you admit to it or not, you have an agenda. And the agenda is to kick this down the road as far as you can. So... we are trying to save lives. That's what we do. I'm a doctor and I'm going to save as many lives as I can. And I'm going to do that through getting the message [out] on Ivermectin... OK. Unfortunately, your work is going to impair that, and you seem to be able to bear the burden of many, many deaths, which I cannot do."
Dr Tess Lawrie: "Would you tell me? I would like to know who pays you as a consultant through WHO."
Dr Andrew Hill: "It's Unitaid."
Dr Tess Lawrie: "All right. So who helped to...? Whose conclusions are those on the review that you've done? Who is not listed as an author? Who's actually contributed?"
Dr Andrew Hill: "Well, I mean, I don't really want to get into, I mean, it ... Unitaid...."
Dr Tess Lawrie: "I think that... It needs to be clear. I would like to know who, who are these other voices that are in your paper that are not acknowledged. Does Unitaid have a say? Do they influence what you write?"
Dr Andrew Hill: "Unitaid has a say in the conclusions of the paper. Yeah."
Dr Tess Lawrie: "OK. So, who is it in Unitaid, then? Who is giving you opinions on your evidence?"
Dr Andrew Hill: "Well, it's just the people there. I don't...."
Dr Tess Lawrie: "So they have a say in your conclusions."
Dr Andrew Hill: "Yeah."
Dr Tess Lawrie: "Could you please give me a name of someone in Unitaid I could speak to so that I can share my evidence and hope to try and persuade them to understand it?"
Dr Andrew Hill: "Oh, I'll have a think about who to, to offer you with a name.... But I mean, this is very difficult because I'm, you know, I've, I've got this role where I'm supposed to produce this paper and we're in a very difficult, delicate balance...."
Dr Lawrie interjects: "Who are these people? Who are these people saying this?"
Dr Andrew Hill: "Yeah... it's a very strong lobby..."
Dr Tess Lawrie: "OK. Look I think I can see [we're] kind of [at] a dead end because you seem to have a whole lot of excuses, but, um, you know, that to, to justify bad research practice. So I'm really, really sorry about this, Andy. I really, really wish, and you've explained quite clearly to me, in both what you've been saying and in your body language that you're not entirely comfortable with your conclusions, and that you're in a tricky position because of whatever influence people are having on you, and including the people who have paid you and who have basically written that conclusion for you."
Dr Andrew Hill: "You've just got to understand I'm in a difficult position. I'm trying to steer a middle ground and it's extremely hard."
Dr Tess Lawrie: "Yeah. Middle ground. The middle ground is not a middle ground... You've taken a position right to the other extreme calling for further trials that are going to kill people. So this will come out, and you will be culpable. And I can't understand why you don't see that, because the evidence is there and you are not just denying it, but your work's actually actively obfuscating the truth. And this will come out. So I'm really sorry... As I say, you seem like a nice guy, but I think you've just kind of been misled somehow."
Dr Andrew Hill: "Well, what I hope is that this, this stalemate that we're in doesn't last very long. It lasts a matter of weeks. And I guarantee I will push for this to last for as short amount of time as possible."
Dr Tess Lawrie: "So, how long do you think the stalemate will go on for? How long do you think you will be paid to [make] the stalemate... go on?"
Dr Andrew Hill: "From my side. OK... I think end of February [2020], we will be there six weeks."
Dr Tess Lawrie: "How many people die every day?"
Dr Andrew Hill: "Oh, sure. I mean, you know, 15,000 people a day."
Dr Tess Lawrie: "Fifteen thousand people a day times six weeks... Because at this rate, all other countries are getting Ivermectin except the UK and the USA, because the UK and the USA and Europe are owned by the vaccine lobby."
Dr Andrew Hill: "My goal is to get the drug approved and to do everything I can to get it approved so that it reaches the maximum...."
Dr Tess Lawrie: "You're not doing everything you can because everything you can would involve saying to those people who are paying you, 'I can see this prevents deaths. So I'm not going to support this conclusion anymore, and I'm going to tell the truth.’"
Dr Andrew Hill: "What, I've got to do my responsibilities to get as much support as I can to get this drug approved as quickly as possible."
Dr Tess Lawrie: "Well, you're not going to get it approved the way you've written that conclusion. You've actually shot yourself in the foot, and you've shot us all in the foot. All of... everybody trying to do something good. You have actually completely destroyed it."
Dr Andrew Hill: "Okay. Well, that's where we'll, I guess we'll have to agree to differ."
Dr Tess Lawrie: "Yeah. Well, I don't know how you sleep at night, honestly."
Ivermectin Part 3: The People Behind the Curtain









You have a gift for writing things clearly, distilling complex subject matter into easy to comprehend language. I hope your work gets much more widely distributed. Thank you 🙏
This is incredible, and shocking. Thank you for YOUR hard work! Wow...just WOW!!!